
We believe that this mode of tuition could be transformational, adding hours to a child's day while providing complete control over the learning process.Įvery course is taught by the best teachers from India's top schools and conducted in an engaging manner to keep students involved. It also provides the complete curriculum at your fingertips for those moments when you need some help at short notice. 88Guru creates a wonderful opportunity for children and parents to bond while engaging in a valuable learning activity. Technology, Connectivity and Social Media are rapidly changing the world of Education and we wish to lead the transformation of the tuition industry in India.Ĩ8Guru is the perfect complement to the current tuition model.
#LEAD ATOMIC MASS NUMBER FULL#
Mission Statement “Empower every student to achieve full potential”Ĩ8Guru has been established with the social objective of making quality video-based learning material available to all Indian students. Tags : #Atomic Number, #Isobars, #Mass Number, #Valency, Atomic Mass, Atoms, Chemistry, isotopes

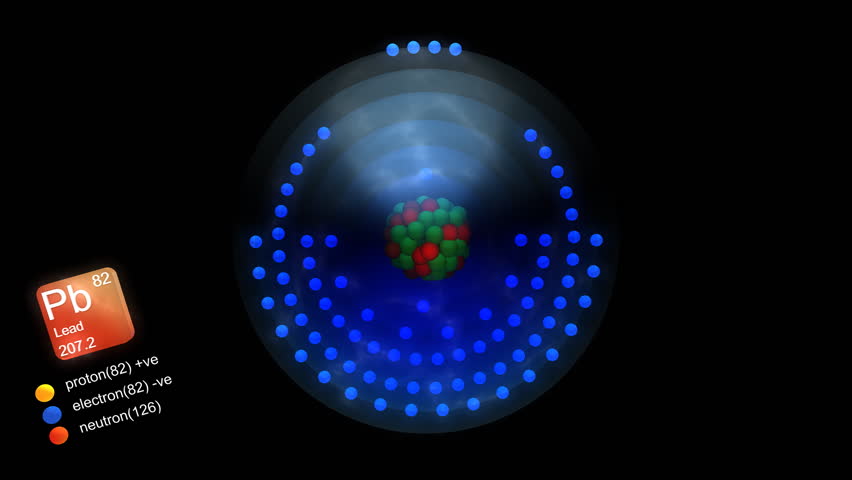
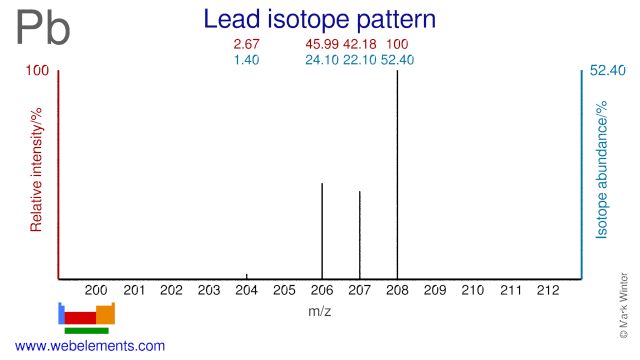
The mass number represents the weight of an atom’s nucleus in atomic mass units. Therefore, an atom’s atomic mass is roughly equivalent to its mass no.
#LEAD ATOMIC MASS NUMBER FREE#
Free Vector Bookmark icon Collection Heart icon Favorite Share icon Share. An electron appears to have very little mass. Chemical symbol with atomic number and atomic mass. The number of neutrons may change, even though the number of protons in such an element’s units remains constant. Even though their mass is so small compared to that of protons and neutrons, electrons are not counted when calculating mass because they have no impact on the value. The atomic number, in contrast, is simply the number of protons. The mass number of an atom’s nucleus is an integer equal to the sum of the nucleus’ protons and neutrons. In order of increasing orbital energy are the following orbitals: The energy of such an electron in a specific atom may be determined solely by the primary quantum number. The maximum capacity for each orbital is two electrons. Orbitals with roughly similar energies have created sub-levels. Orbitals are the name for those sections. When an electron reaches a certain energy level, it is more likely to be found in these regions than in other regions. of isotopes seems to be the same.Įlements are classified as monovalence, divalence, and trivalence based on their valency. of electrons does have a direct relationship with valency. of neutrons inside an atom seems to not affect the mass no. of neutrons in an atom does not impact its atomic no. is always greater than that of the mass number. The mass number has always been less than the atomic no. The electronic arrangement of such an atom could be used to evaluate its valency. within an atom is the total of its protons as well as neutrons. Chemistry Portal v t e Dmitri Mendeleev published a periodic table of the chemical elements in 1869 based on properties that appeared with some regularity as he laid out the elements from lightest to heaviest. The greatest amount of electrons that even an atom could lose, gain, as well as share, in addition to getting stable is referred to as valency.Īn atomic no. What is the difference between Valency, Atomic number and Mass number Valency Its nucleus contains twenty neutrons and seventeen protons. This has typically been accomplished by simultaneously adding both neutrons and protons.įor instance,\(Cl^ \) appears to have a mass number of 37. To represent it, the letter “A” is frequently used. Atomic mass units are used to measure this. The mass number refers to the total number of protons and neutrons in such an atom. Rutherford proved that an atom’s nucleus, which is composed of protons and neutrons, contains perhaps the majority of the atom’s mass.


 0 kommentar(er)
0 kommentar(er)
